In reply to:
Michael Shellenberger
@shellenberger
·
147d
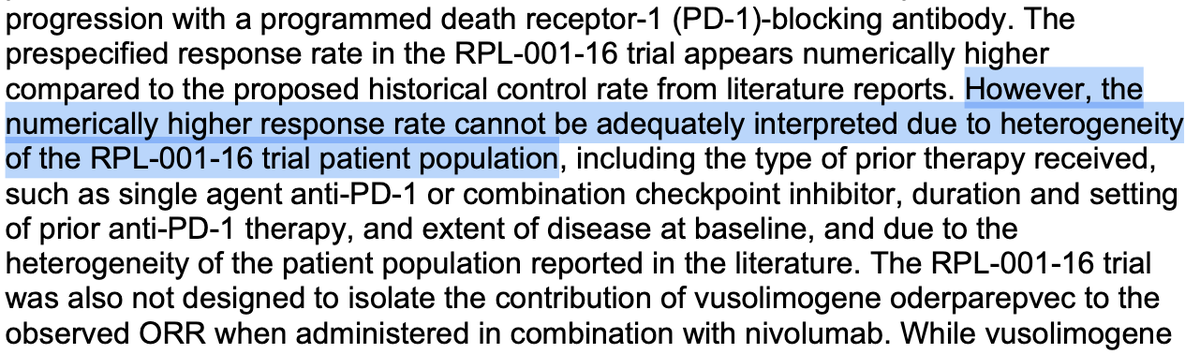
The statement critiques the FDA's decision-making process regarding drug approvals for rare diseases, suggesting that the agency is overly cautious and potentially hindering access to life-saving treatments. The accompanying images provide context for the FDA's decisions, highlighting concerns about trial heterogeneity, lack of meaningful treatment effects, and manufacturing deficiencies. The conversation involves public discourse on drug approval standards and patient access to experimental treatments.
- The statement raises concerns about potential harm due to lack of access to treatments, but it does not provide a balanced view of the FDA's rationale, which could mislead the public. [-1]Principle 1:I will strive to do no harm with my words and actions.
- The statement could promote understanding by highlighting the complexity of drug approvals, but it lacks empathy for the FDA's responsibility to ensure safety and efficacy. [-1]Principle 3:I will use my words and actions to promote understanding, empathy, and compassion.
- The statement engages in criticism of the FDA but does not acknowledge the detailed reasons for the rejections, which are provided in the images. [-1]Principle 4:I will engage in constructive criticism and dialogue with those in disagreement and will not engage in personal attacks or ad hominem arguments.